About us
Scroll down to read more about us
Scroll down to read more about us
Read more about our service
How to contact us
Disturbed intestinal flora caused by e.g. antibiotics can lead to bacterial infection with potentially life-threatening diarrheal disease caused by Clostridoides difficile infection; CDI.15 000 people are at risk every year in Sweden only.
Faecal Microbiota Transplants (FMT) is the most efficient treatment to quickly restore a disturbed intestinal flora and regain resilience from opportunistic bacteria, such as CD.
Conventional FMT is expensive and cumbersome for healthcare providers and unpleasant for the patient - it is therefore vastly underutilized.
Bactaviva offers outsourced capsule-based FMT, a cost-efficient and convenient administration for healthcare providers and with a markedly improved patient acceptance.
Clinics that have healthy donors can use our service to produce capsules of their healthy microbiome for convenient and safe FMT.


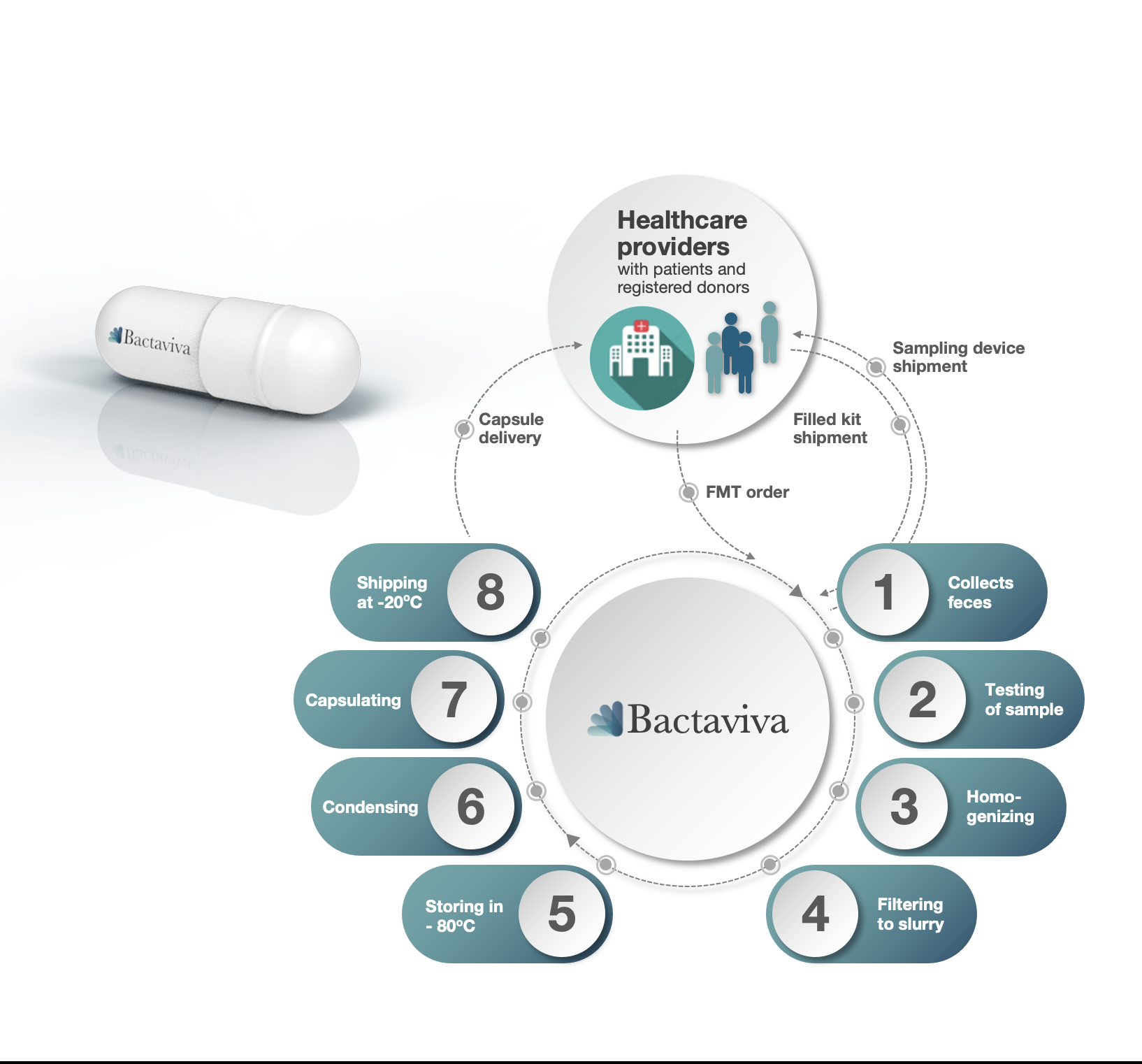
Bactaviva offers healthcare providers the opportunity to efficiently and conveniently use FMT treatment by preparing feces in capsules to prevent and treat illness caused by disturbed intestinal flora.
Our conventional FMT is done with donor feces in capsules and used reactively to an existing illness caused by disturbed intestinal flora.
However, when your own intestinal flora is used (autologous FMT - AFMT), it becomes possible to treat preventively to restore the intestinal flora prior to infections occurring. This would be applicable for planned treatments that involves antibiotics, cytostatic and radiation therapy. This minimizes the risk of severe diarrheal diseases, resistant bacteria and dysfunctional drug metabolism as well as mitigating antibiotic resistant bacteria.
Utilizing AFMT in this way is a new approach. With one's own healthy flora, this can be done with great safety(1) and thus prophylactically, as no infectious agents unknown to the individual are transmitted.
1 Stefansson M, Bladh O, Flink O, Skolling O, Ekre HP, et al. (2023) Safety and tolerability of frozen, capsulized autologous faecal microbiota transplantation. A randomized double blinded phase I clinical trial. PLOS ONE 18(9): e0292132. https://doi.org/10.1371/journal.pone.0292132